Biophysics
We use a range of techniques when we study protein conformation, stability, interactions etc. The methods presented below are used on a daily basis in the lab.
Main content
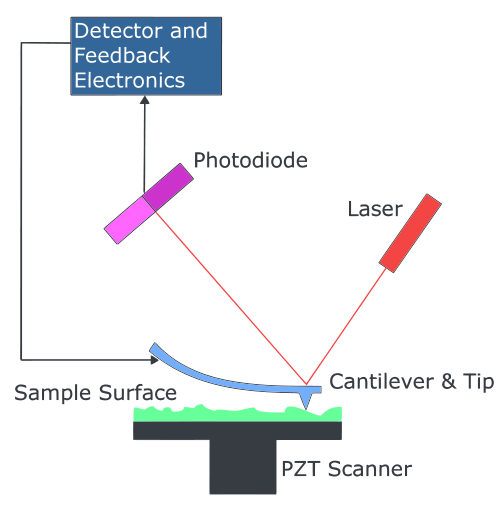
AFM
Atomic force microscopy (AFM) is a high resolution imaging method that can be used to study the surface topography in atomic resolution and the adhesion of e.g. nanoparticles, macromolecules and cells to a surface. The principle is based on a laser beam deflection system.
In our lab the MFP-3D-BIOTM (Asylum research) is mainly used to explore distortions on a membrane due to peptide/protein interactions. Therefore samples are prepared with the Langmuir monolayer technique in such a way that the lipid headgroups are oriented towards the supporting substrate (mica) and the interacting polypeptides are expected to be trapped between the mica and the lipid monolayers or, alternatively, intercalated in the lipid film.
SPR
Surface plasmon resonance (SPR) is a powerful, label-free technique that measures biomolecular interactions in real time. In addition, it can deliver values of the kinetic constants kon and koff and the affinity constant (Ka= 1/Kd, where Kd is the dissociation constant). It is based on optical technology where mass concentration of biomolecules in close proximity to a sensor chip surface is measured.
In our lab the Biacore 3000 (Biacore AB) is used to investigate protein–protein and protein–membrane interactions. For studying binding to proteins, the CM5 sensor chip, with a carboxymethylated dextran matrix covalently attached to a gold surface, is employed. Proteins/peptides are immobilized covalently to the CM5 chip by the standard primary amine coupling reaction. On the other hand, liposomes are immobilized on L1 sensor chips with lipophilic groups covalently attached to the carboxymethylated dextran matrix.
DLS
Dynamic light scattering (DLS) is a well-established method to characterize nanoparticles and biological macromolecules including proteins and polymers in the submicron region. This technique is mainly used to determine the size or size distribution, the molecular weight or the zeta potential of a particle. DLS determines particles movement (Brownian motion) by measuring dynamic fluctuations of intensity scattered light and relates this to the size of the particles.
In our lab the Zetasizer Nano ZS (Malvern Instruments) is used to determine the size of nanoparticles and liposomes and to investigate conformational stability of a protein and their tendency to oligomerize and aggregate over time or depending on temperature.
CD
Circular Dichroism (CD) is a type of absorption spectroscopy which measures the difference between the absorption of left and right handed circularly-polarized light as a function of wavelength. For the purpose of CD on protein and peptides, CD spectra are divided into two different regions, called far and near UV. The far-UV spectral region lies between 190 nm and 250 nm. At these wavelength changes in the secondary structure can be observed while the tertiary structure is determined in the near-UV range (250 nm - 350 nm).
In our lab the Jasco J-810 spectropolarimeter is used to obtain information about the secondary and tertiary structure of peptides and proteins, and to study their thermal unfolding.
ITC
Isothermal titration calorimetry (ITC) is a technique used to characterize interaction between binding partners. The method is label-free, only requiring pure preparations of the components in the system under study. As a binding event occurs, heat is either released (exothermic reaction) or absorbed (endothermic reaction) and this is directly measured in the ITC when one binding-partner is titrated into another. A full thermodynamic characterization can be obtained, including the stoichiometry of binding and affinity constants.
In our lab the ITC-200 (Microcal, GE Healthcare) is mainly used to investigate protein-ligand interactions – both with natural ligands and potential chaperones for misfolding mutations.
Fluorescence
Intrinsic fluorescence may be used to investigate small conformational changes as tryptophan (Trp) and tyrosine (Tyr) are naturally occurring fluorophores that are very sensitive to the polarity of its immediate environment. Trp is the dominant intrinsic fluorophore, and has a maximum emission of 352-355 nm when fully exposed to water, and near 310 when completely buried within a hydrophobic protein core. The fluorescence may be changed by e.g. positively charged groups like –NH3+, –COOH and protonated histidine residues. Interactions such as van der Waals interactions or hydrogen bonding between solvent molecules and the fluorophore, as well as changes in the solvent nature or composition, may also cause changes in the fluorescence response.
In our lab, we use a Cary Eclipse Fluorescence Spectrophotometer (Agilent Technologies) with temperature control.