Hangin’ out at the Golgi
Many proteins have the Golgi apparatus as their favorite hangout place. For N-terminal acetyltransferases (NATs), however, this is a rather unique characteristic as only one NAT, Naa60, was shown to localize to intracellular organelles.

Main content
Naa60 and the means by which it associates with the Golgi membrane caught the interest of researchers at The Department of Molecular Biology who recently presented their findings in The Journal of Biological Chemistry.
Hangin’ out by means of peripheral membrane binding
The Golgi, or the Golgi apparatus, is a membranous organelle that functions in the modification and transport of proteins as part of the cellular secretory system. Inside the cell, proteins may localize to the Golgi either as soluble molecules in its interior space, the lumen, or attached to the Golgi membrane. Membrane attachment may be achieved by parts of the protein structure completely traversing the membrane bilayer or by peripheral membrane binding at either the lumenal or cytosolic side. Researchers in Thomas Arnesen’s group (the NAT group) and colleagues at The Department of Molecular Biology, UiB, have now characterized the membrane-interacting properties of a recently discovered enzyme, Naa60, that associate at the cytosolic side. Naa60 “hangs” on the surface of the Golgi by means of structurally organized parts that interact with and partially integrate into the membrane lipid bilayer (Main figure).
Naa60: An unconventional NAT
This finding is particularly interesting, since Naa60 is the first enzyme of its kind shown to localize to intracellular organelles and membranes. The other members of this enzyme family, the N-terminal acetyl transferase (NAT) family, are mainly found in the cytosol where they associate with ribosomes, the protein-producing factories of our cells. The role of all NATs is to facilitate a chemical modification of substrate proteins by catalyzing the attachment of an acetyl group to their N-terminal end. From the ribosomal position NATs can perform this task while the substrate proteins are being produced, i.e. during the translation from RNA to protein. This type of co-translational protein modification is perceived as the general mode of action for NATs. The newly published work by the NAT group therefore reveals a new concept, since Naa60’s Golgi localization is strongly suggestive of a post-translational enzyme activity.
Naa60’s peripheral membrane binding involves two amphipathic a-helices
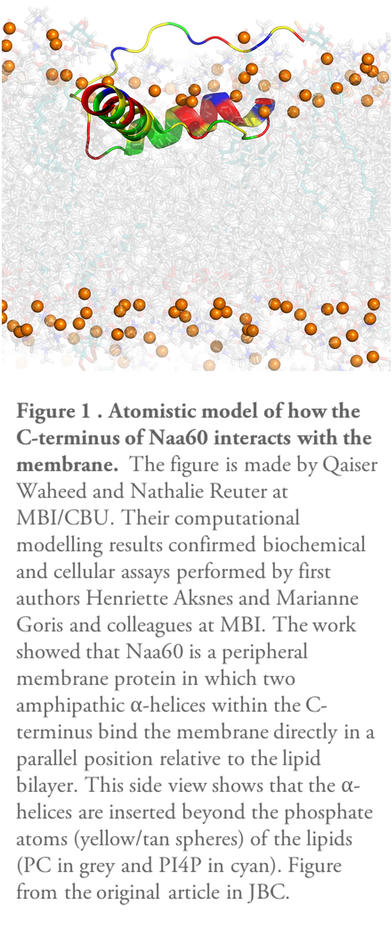
In their recent publication in The Journal of Biological Chemistry (JBC), the researchers in Thomas Arnesen’s research group looked in detail at the intrinsic properties of Naa60 that facilitate this unique subcellular localization. Previous work had indicated the C-terminal part of Naa60 to be responsible for the organellar localization. However, X-ray crystallography studies investigating the 3D structure of Naa60 were unable to resolve the C-terminal tail of Naa60. Through collaboration with Nathalie Reuter’s research group at Computational Biology Unit, Department of Informatics, UiB, the current work combined computational modeling with biochemical assays and cellular localization studies to investigate the secondary structure and membrane interacting ability of Naa60. The results show that Naa60 is a peripheral membrane protein in which two amphipathic alpha-helices within the C-terminus bind the membrane directly in a parallel position relative to the lipid bilayer via hydrophobic and electrostatic interactions (Figure 1)
Implications and future Naa60 endeavors
The work behind the recent article is founded on previous work of the NAT group in which Naa60 was established as a Golgi-residing NAT that acted only on transmembrane substrate proteins. Future work by the NAT group will endeavor to reveal the mechanistic action of Naa60 towards these substrate proteins and the consequences of their modification that might be linked to Naa60’s function in maintaining Golgi structural organization. The researchers in the NAT group hope that this will enlighten some aspects of how the NAT enzymes perform their vital tasks in the human body and how NAT malfunctioning relates to disease.
The study is published in the scientific journal The Journal of Biological Chemistry (JBC) February 14th, 2017 and is part of Marianne Goris’ PhD work.
The NAT group’s research receives funding from Bergen Research Foundation, The Research Council of Norway, The Norwegian Cancer Society and Western Norway Regional Health Authority.