Why evolution invented a paradoxical enzyme that eats up vitamin B3
Mathematical modeling and systems biology explain the evolutionary transition from a four-step to a two-step pathway for the synthesis of NAD from vitamin B3.

Main content
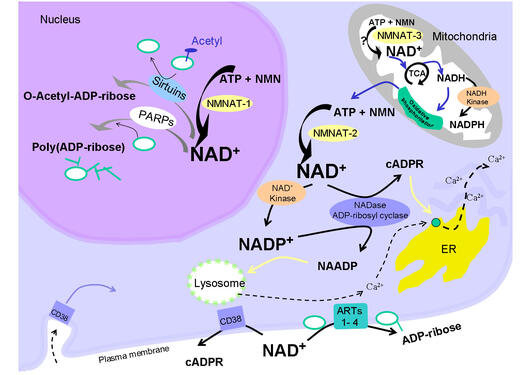
Water, ATP, and DNA are likely nominated by many researchers as the top-three candidates for the molecules of life. If you ask members of Mathias Ziegler´s research group at the Department of Biomedicine, they will certainly add nicotinamide adenine dinucleotide (NAD) to that list.
NAD (PubChem ID 5892) is undoubtedly a hidden champion in biochemistry textbooks. Like a rechargeable battery, it serves as an electron carrier, when chemical energy stored in the nutrients is eventually converted into ATP, the universal energy currency that cells need to live. There is no life without this function of NAD, and therefore, this molecule is present in all organisms from bacteria to humans. With increasing organismal complexity throughout evolution, additional NAD-dependent pathways have emerged that control vital processes such as DNA repair as well as cell cycle and gene regulation, which are important in human health and disease. These evolutionary more recent functions of NAD lead to a constant consumption of this small molecule and therefore, demand for continuous biosynthesis NAD from vitamin B3.
Three major observations…
Using phylogenetic analyses combined with mathematical modelling, a consortium of laboratories from Norway, Germany and the UK (lead-managed by Ines Heiland from the Institute of arctic and marine biology at the Arctic University of Norway in Tromsø) pursued a computational approach to recapitulate the evolution of vitamin B3 metabolism and made three interesting observations:
- First, the researchers found that an evolutionary switch from a four-step pathway of NAD biosynthesis in lower organisms to a two-step pathway in higher organisms coincides with the emergence of a seemingly paradoxical enzyme named nicotinamide N-methyltransferase (NNMT). At the first look, NNMT behaves as a “bad guy” in metabolism: It virtually prevents vitamin B3 from being used as NAD precursor! So why did nature create an enzyme that removes a compound that cells clearly need to produce a vital molecule?
- Second, whilst analyzing NAD metabolism throughout the tree of life, the researchers found that the “paradoxical” enzyme NNMT entered the stage at a time where higher organisms developed novel mechanisms that rely on the consumption of NAD in order to control complex vital functions.
- Third, they found that, when NNMT entered the stage, evolution invented a “modern” form of the enzyme nicotinamide phosphoribosyltransferase (NamPT), which contains a handful more amino acids than the “vintage” enzyme present in lower organisms. This evolutionary adaptation attracted the researchers´ attention, since NamPT is a key player in NAD biosynthesis from vitamin B3 and therefore implicated in therapeutic concepts.
…and a surprisingly simple explanation
The researchers in Tromsø developed mathematical models to analyze NAD-dependent pathways and to simulate enzymes involved in the biosynthesis and the consumption of NAD. With the help of these models, they could provide a rather simple explanation for the emergence of NNMT: NAD-consuming pathways produce nicotinamide as a breakdown product, and nicotinamide is a feedback inhibitor of these pathways. NNMT facilitates high NAD turnover resulting from complex NAD-dependent pathways by preventing the accumulation of nicotinamide. So far so good. But what about the evolutionary adaption of NamPT? Why is the “modern” NamPT equipped with ten additional amino acids?
In order to answer this question, the collaborators from Tromsø sought the help of the laboratory of Mathias Ziegler at the Department of Biomedicine.
From dry lab to wet lab at the Department of Biomedicine…
Dorothée Houry and Marc Niere aimed at elucidating the role of the ten additional amino acids in the “modern” NamPT using the human enzyme as experimental model. They asked, how key properties of the human NamPT change, when the enzyme is converted back to its “vintage” precursor. To this end, they took the ten additional amino acids out of the human enzyme and then measured its ability to “prepare” nicotinamide for the biosynthesis to NAD: They demonstrated that this modified human NamPT converts nicotinamide far less efficiently than the regular human NamPT. In other words, the engineered human NamPT mimicked the “vintage” enzyme from bacteria. These experimental data fully matched with the phylogenetic model developed in Tromsø: With the emergence of complex NAD-dependent processes, nature optimized NamPT to support NAD biosynthesis from nicotinamide as efficiently as possible.
…and further to bedside?
While these findings are fascinating to evolutionary biologists and biochemists, they have potential medical implications. Manipulating the activity of NamPT is implicated in therapeutic concepts, while NNMT expression is upregulated in some cancer types. As such, the kinetic interplay between NamPT and NNMT needs to be considered in strategies aimed at starving cancer cells from energy.
This research is published in the Proceedings of the National Academy of Sciences of the U.S.A. (PNAS) in August 2019. https://www.pnas.org/content/116/32/15957