Protein N-terminal acetylation
From molecular mechanisms to human disease
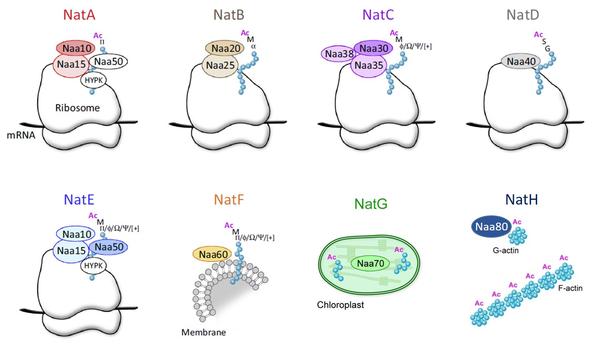
Most proteins are chemically modified in the cell and such modifications are often crucial for the protein’s ability to carry out a function. N-terminal acetylation one of the most common modifications in eukaryotes. It is catalyzed by N-terminal acetyltransferases (NATs) which are linked to cancer, genetic syndromes, and regulation of human metabolism.
Translational Protein Research group (Arnesen lab) is part of the Systems Biology and Translational Cell Signaling research unit at the Department of biomedicine.