Research at the Retinal Microcircuits Laboratory
The Retinal Microcircuits Laboratory is interested in the cellular and molecular basis of synaptic transmission and synaptic integration in the central nervous system. Our main goal is to understand and characterize the synaptic and cellular mechanisms employed by identified neurons and specific microcircuits for signal processing.

Main content
Description of research interests:
Our lab is interested in the cellular and molecular basis of synaptic transmission and synaptic integration in the central nervous system. Our main goal is to understand and characterize the synaptic and cellular mechanisms employed by identified neurons and specific microcircuits for signal processing. The primary technique is that of targeted patch-clamp recording of visually-identified neurons using voltage clamp, current clamp and dynamic clamp recording configurations. This electrophysiological work is currently performed using an in vitro slice preparation of the rat retina, a preparation that offers the possibility to combine modern electrophysiological techniques with the use of natural stimuli to activate specific neuronal circuits.
Current projects include:
1) Performing combined multi-photon excitation imaging and patch-clamp recording to study healthy tissue and processes of neurodegeneration
2) Performing Ca2+-imaging in retinal slices
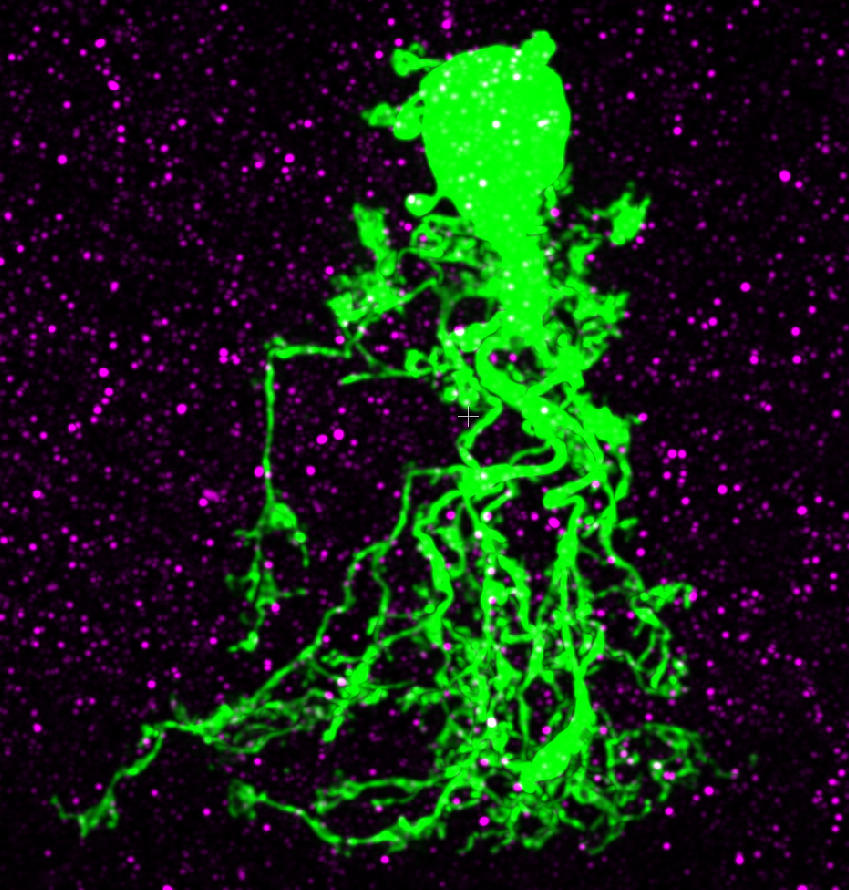
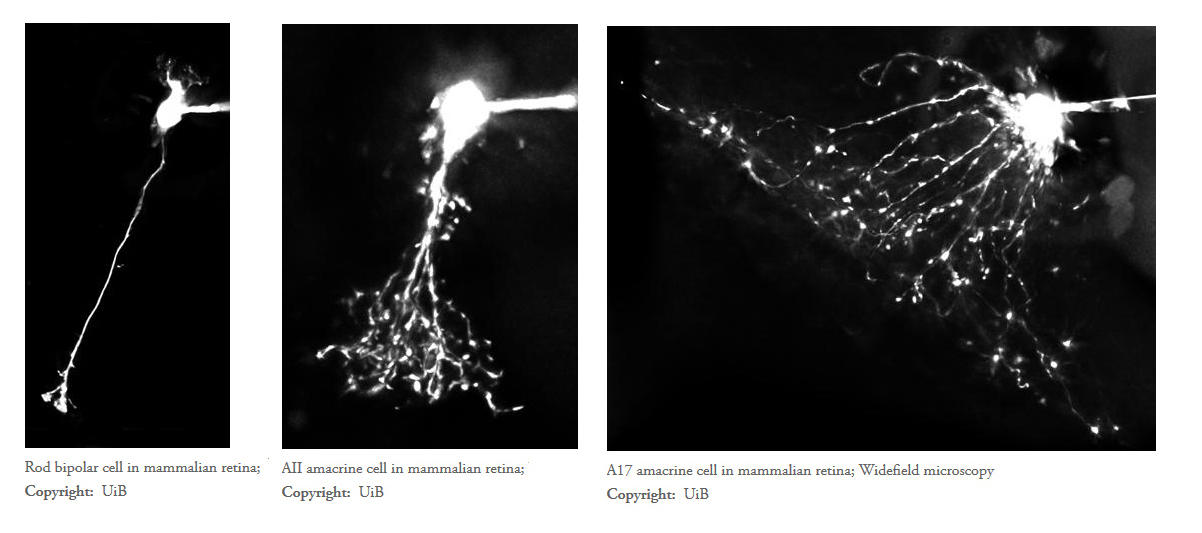
3) Performing quantitative morphological reconstructions of single neurons by computer-aided manual tracing of image stacks acquired with multi-photon excitation microscopy
4) Constructing compartmental models of single neurons to study passive and active properties involved in signal processing
4) Performing simultaneous multi-electrode recordings from neurons in specific microcircuits within the inner retina
5) Studying the location and functional properties of NMDA receptors in retinal microcircuits
6) Utilizing dynamic clamp to artificially insert synapses and conductances into neurons, enabling us to study the mechanisms underlying the dynamic properties of neurons and microcircuits.
7) Performing confocal and STED microscopy to localize receptors and synaptic proteins to individual neurons