Anti-CNPase nanobodies light up myelin
CNPase is an enzyme present at high levels in myelinating cells. Novel nanobody tools developed in an international collaboration were used to identify binding epitopes at atomic resolution and visualise myelin. Additionally, the nanobodies were used as intrabodies to bind to CNPase in living cells. These nanotools will be valuable for future research on myelin and its molecules.

Main content
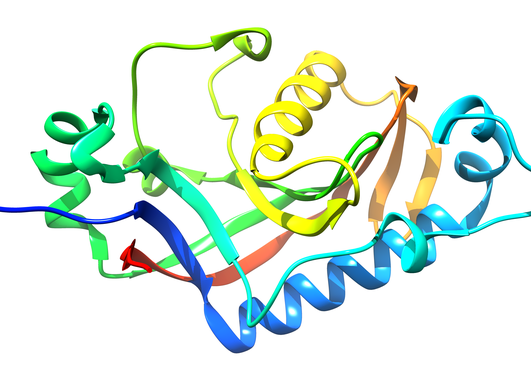
In a multinational collaboration involving Norway, Germany, Finland, Belgium, and the United States, we developed a set of nanobodies – small proteins derived from the antibodies of alpacas – that bind the myelin enzyme CNPase with ultrahigh affinity. These nanobodies have numerous advantages compared to classical antibodies used in molecular biology, such as their small size, easy recombinant expression, straightforward labelling, as well as expression in live cells as intrabodies. The five nanobodies have different epitopes, as shown by X-ray crystallography (see Figure); yet, they all bind CNPase with nanomolar affinity. One of the nanobodies inhibits the enzymatic activity of CNPase, making it a prospective tool for enzyme inhibition in living systems. The nanobodies can be used for labelling CNPase in both teased nerve fibers and brain slices, and the nanobodies bind and mark endogenous and recombinant CNPase in cell culture. The findings pave way for further experiments, such as the structural characterisation of full-length CNPase using X-ray crystallography or cryo-EM. The nanobodies will be invaluable tools for myelin research in general, given that CNPase is one of the most used myelin markers in various in vitro and in vivo studies.
Original article:
Markússon et al., J Neurochem, in press
Earlier related work from our group:
Snaidero et al. (2017), Cell Rep