From MOL231 Project to Published Article
Ine and Emilie were attending a project course priming them for lab work. They ended up co-developing a method to quality control a cell model which is used by thousands of researchers worldwide.

Main content
Through our work in the course Project in Molecular Biology (MOL231), we participated in fine-tuning and documenting a quality control protocol for the increasingly popular CRISPR/Cas9-friendly cell line HAP1. In our recent paper together with supervisors Henriette Aksnes and Tobias B. Beigl, we reveal how HAP1 ploidy status affects various cellular characteristics and share our effective solution to avoid ploidy differences as an interfering variable.
HAP1 cells and ploidy instability
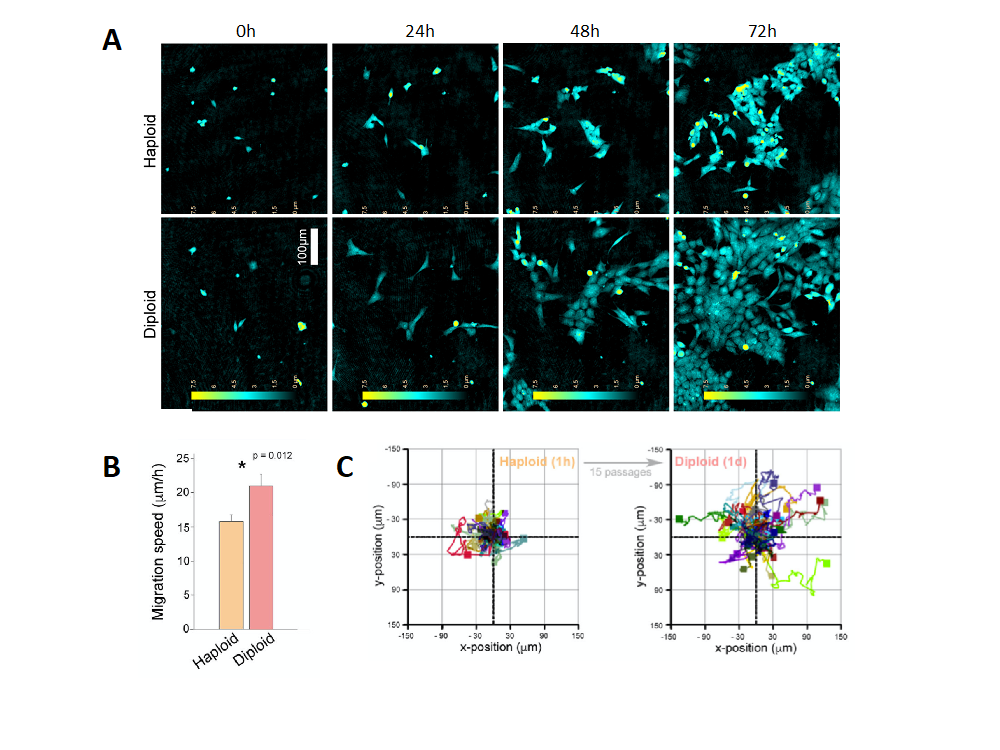
Unlike diploid cells, which have chromosomes existing in pairs with one copy from each parent, haploid cell types like HAP1 contain only a single genome copy. This presents a major advantage when generating genetic mutants because only one allele is needed to be targeted by the CRISPR/Cas9 machinery. However, while working with HAP1 knockouts, our supervisor Henriette observed that the haploid state of HAP1 is unstable and that these cells spontaneously diploidize with time. Through further investigations, including holographic imaging by the HoloMonitor M4, we detected that there were several basic differences between haploid and diploid HAP1 cells. These included a difference in cell-size, cell migration speed and cell confluency (Figure 1).
To eliminate ploidy as an interfering variable in experiments we, together with Henriette and Tobias, began to outline an effective and affordable protocol for passaging cells until the diploid state and control for ploidy status by flow cytometry. Importantly, we also successfully demonstrated that diploid cells can be sorted from culture at an early passage, which saves both time and money on cell culture work.
Controlling HAP1 ploidy status by flow cytometry
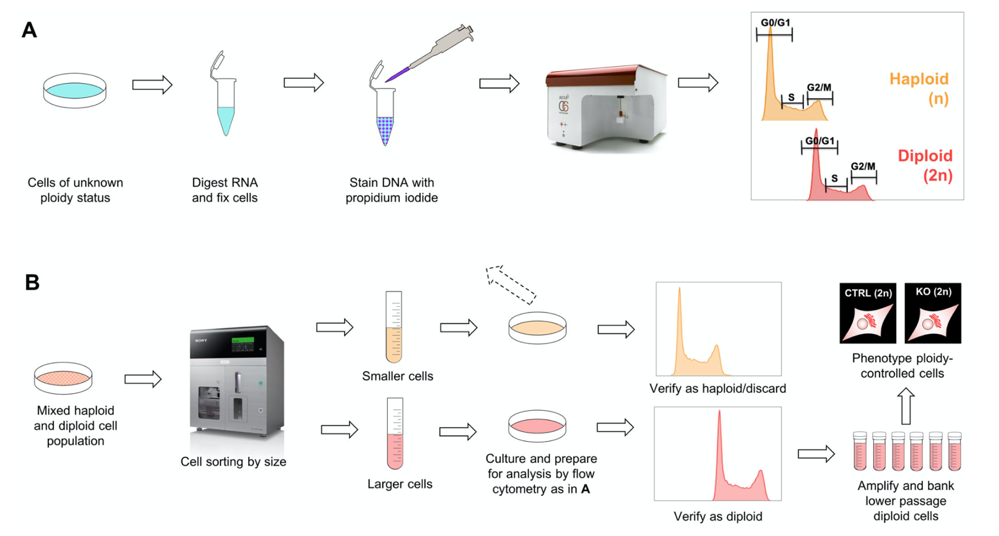
To control for ploidy status, we used an already established approach in which the DNA content of propidium iodide (PI)-stained cells was measured by flow cytometry. Using this approach, we controlled several knockout cell lines at various passages. As our amount of ploidy controlling data increased, we observed a tendency of cells to start diploidization around passage 10 and reach a pure diploid state around passage 20-30.
Fast forwarding to the diploid state by size-based cell sorting
As diploid HAP1 cells are larger than their haploid counterparts, we employed a cell-size based sorting approach using a Sony SH800 flow cytometer. Next, we analyzed both output cultures for ploidy status. We concluded that by using this size-based cell sorting strategy, you can obtain a pure diploid cell culture at passage 18 and possibly earlier. Our streamlined quality control protocol is summarized in Figure 2.
Undergraduate students can make valuable contributions to your research project
By the combined efforts of students and researchers on different junior career levels we were able to achieve our goal of publishing our effective and affordable solution to the spontaneous diploidization of HAP1 (Beigl, Kjosås, Seljeseth, Glomnes & Aksnes, 2020). Even though universities all over are filled with highly motivated, eager-to-learn students, most undergraduates in natural sciences never get the opportunity to take part in a research project. Because they lack experience and scientific knowledge, many principal investigators believe that training of undergraduates is just too time consuming. However, as students gain more experience, we can help to speed things up, introduce new perspectives and ask questions that most likely will benefit the project.
By our participation in MOL231, the two of us got the opportunity to follow a real research project through its different phases. We had valuable hands-on experience with cell culturing, flow cytometry and holographic imaging, and learned to understand the importance of cell line quality control. We remain way thankful to the Arnesen lab and the team lead by Henriette for including us into their professional and friendly work environment and for investing the time to train and guide us.