Molecule of the Month (August 2012): "Tris"
Tris(hydroxymethyl)-aminomethane (short: “Tris”) by Marc Niere
Hovedinnhold
This article was awarded the best contribution to MBInytt's "Molecule of the Month" column for 2012.
Probably everyone at the MBI, who did experiments during the past …hours, used this compound. Life scientists often associate Tris simply with the preparation of “a buffer”. It is omnipresent in our pipet tips, yet, unknown to many of us and, hence, a classic for the “What am I actually pipetting?” issue.
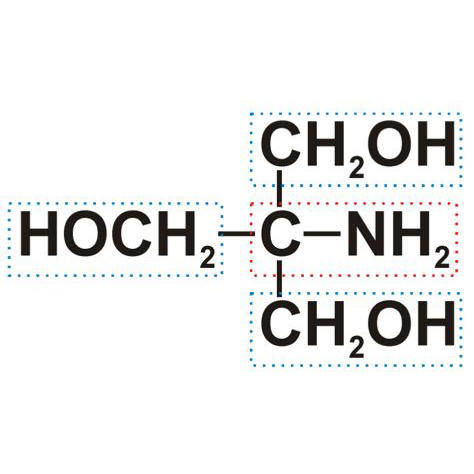
The relatively simple organic compound (Figure) is synthesized from highly explosive nitromethane and carcinogenic formaldehyde. Despite the hazardous precursors, Tris is a friendly and nearly harmless compound. Beware, however, that it can be irritant, so don´t use Tris-solutions as beauty cream and if you feel the desire to put your head directly into the Tris can, then at least close your eyes and stop breathing.
The molecular formula C4H11NO3 suggests that a total of 6.023x1023 molecules add up to 121.136 g. This molar mass (granted, 121.14 g/mole…) is probably among the first ones young master students at the MBI bear in mind.
Particularly its high buffer capacity between pH 7.1 and 9.0 makes this primary amine pretty suitable in biological experiments. In other words, within this pH range you can dump quite a lot of acid or base into your Tris-solution without substantially changing its [H3O+]. Moreover, Tris is cheap, water soluble and does not affect the activity of most enzymes. Though, Tris displays some unwanted features. Tris buffers are sensitive to temperature changes (50 mM Tris pH 7.4 at 25 °C changes to pH 7.99 at 4 °C and 7.06 at 37 °C). Furthermore, dilution alters the pH of Tris solutions, which should be considered when preparing stock solutions. It is also not a good idea to expose cells to Tris, since it permeates membranes and is toxic for many mammalian cells. Moreover, the nucleophilic amino group makes Tris unsuitable in aldehyde-based fixation solutions and also prohibits Tris in diethylpyrocarbonate-treated solutions. Finally, “Tris-contaminated” protein structures were reported.
Unaware of some of these pitfalls that one may encounter when using Tris, the author must confess to have messed up at least two experiments during his professional career. That is, it is a good concept to always try to understand what exactly is in your pipet tip.