Mechanism of a cell motility regulator
Actin is the most abundant protein in human cells and is involved in numerous functions including steering cellular architecture, cell motility and cell division. Recently, UiB researchers identified NAA80 as a long-sought actin regulator. Now, the structure of NAA80 bound to actin and profilin reveals its mechanism of action.

Main content
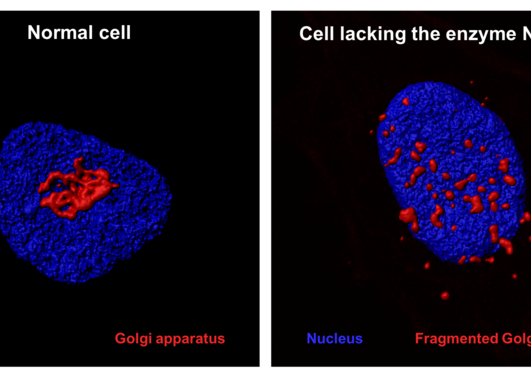
Actin is an essential component of the cytoskeleton of human cells and is involved in a plethora of functions. For several decades it was known that actin's N-terminus was chemically modified in cells by an acetyl group, but only two years ago the actin N-terminal acetyltransferase NAA80 was identified by UiB researchers. NAA80 mediated N-terminal acetylation of actin strongly impacted cytoskeleton dynamics and cell motility thus stressing the biological importance of this modifier.
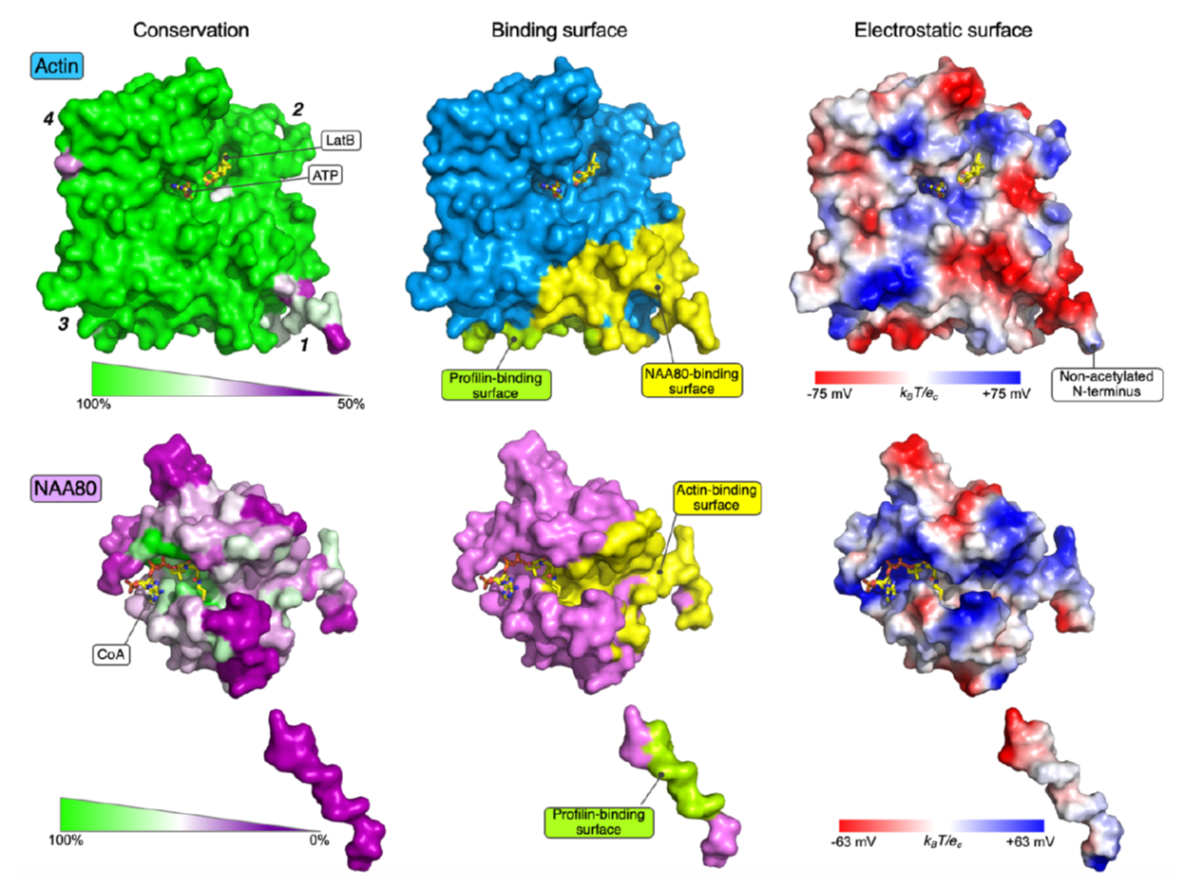
In human cells there is a machinery of seven N-terminal acetyltransferases (NATs) catalyzing N-terminal acetylation of totally 80% of the human proteome. Most NATs therefore have a large number of substrates and many operate on the ribosome to carry out co-translational acetylation. In contrast, actin has a dedicated NAT, NAA80, which acts post-translationally. However, detailed knowledge on how this unique targeting was accomplished has remained unknown. The actin N-terminus is highly acidic and the structure of NAA80 uncovered a catalytic groove packed with positive charges perfectly matching this acidic actin stretch. Still, NAA80 has to distinguish actin from many other cellular proteins with similar N-termini. The strong selectivity of NAA80 for actin was revealed by in vitro binding studies where it was uncovered that actin was capable of forming a tight complex with NAA80 and further that the known partner of monomeric actin, profilin, was a potential part of this. The trimeric structure of actin-NAA80-profilin was solved in the Dominguez lab at the University of Pennsylvania, and uncovered the extensive binding surface between actin and NAA80 and further a direct interaction between NAA80 and profilin thus demonstrating a direct physical connection between all three proteins. Biochemical and molecular biology investigations in the Arnesen lab at UiB were carried out by current lab member Rasmus Ree, and alumni Adrian Draciz and Marianne Goris. These experiments defined that the specific interactions between NAA80 and actin/profilin were critical for cellular actin acetylation by NAA80. Thus, the current data explains how NAA80 uniquely acts on actin and represents the first structure of a NAT bound to its entire substrate.
The Arnesen lab acknowledges support from Helse Vest, the Norwegian Cancer Society, the Research Council of Norway and the European Research Council (ERC). Rasmus Ree is a Postdoctoral candidate supported by the Medical Faculty at UiB.