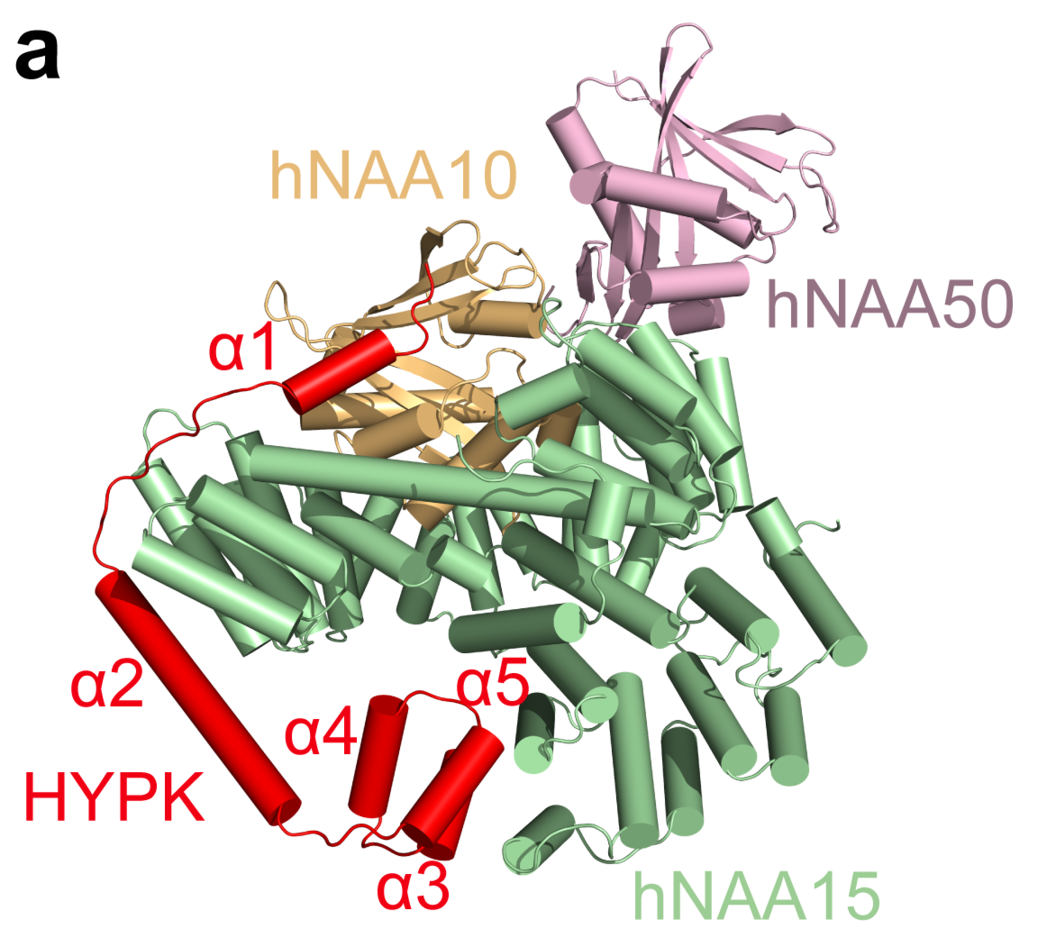
The structure of an enzyme complex upregulated in cancer
In human cells, N-terminal acetylation is among the most common protein modifications. Now, researchers at the University of Pennsylvania and the University of Bergen have revealed the structural and biochemical properties of the major molecular machine involved in this process. Cancer cells require this enzyme for survival and proliferation.

Main content
Approximately half of all N-terminal acetylation events in humans is catalyzed by an enzyme complex called N-terminal acetyltransferase A (NatA). Originally found upregulated in thyroid cancers by UiB researchers 20 years ago, and biochemically defined 15 years ago, a near-complete molecular understanding of this enzyme is now revealed. Research groups at the University of Pennsylvania and the University of Bergen solved the structure and biochemical features of the human tetrameric NAA15-NAA10-NAA50-HYPK complex. NAA15 acts as an anchor at the ribosome and puts the two catalytic subunits NAA10 and NAA50 in appropriate positions to carry out N-terminal acetylation of the nascent polypeptides that emerges from the ribosome. HYPK on the other hand slows down the catalytic reaction, probably facilitating quality control of the acetylation process. Professor Ronen Marmorstein’s lab at UPenn in Philadelphia solved the structure of this holoenzyme by cryo-EM which provides near atomic resolution of all subunits. In Bergen, Ph.D. candidate Nina McTiernan in the Arnesen lab at the Department of Biomedicine elucidated the activity of different enzyme complexes in human cells in order to understand the contribution of the different subunits.
The NAA15-NAA10-NAA50-HYPK complex co-translationally acetylates nascent peptides with N- termini of either initiator methionine maintained (called NatE – reaction catalyzed by NAA50) or cleaved (called NatA – reaction catalyzed by NAA10), which accounts for the largest number of substrates among all NATs. Given that this complex or its subunits are functionally altered in many human diseases such as different cancers (see review) the studies presented here provides an important molecular scaffold for potential therapeutic development.
Read the full paper here.
The Arnesen lab acknowledges support from Helse Vest, the Norwegian Cancer Society, the Research Council of Norway and the European Research Council (ERC). Nina McTiernan is a Ph.D. candidate supported by the Medical Faculty at UiB. Parts of the work was carried out at the Proteomics Unit at University of Bergen (PROBE).